Quality Control Testing of Packaging Materials
Introduction
Pharmaceutical packaging is an important part of a pharmaceutical industry.
Packaging not only affects the quality and stability of any pharmaceutical product but also helps in the product identification.
It provides
It provides
- An adequate degree of protection
- Minimize the loss of content
- Should not interact physically or chemically with the contents in a way that will alter their quality to an extent beyond the limits given in the individual monograph, or present a risk of toxicity.
- Protection
- Presentation
- Identification
- Information and
- Convenience to encourage compliance with a course of therapy.
- Container
- Closure
- Carton or Outer and
- Box
- Glass
- Plastic
- Metal
- Paper
- Cork
- Glass
- Plastic
- Metal
- Rubber
There are various tests for determination of quality, integrity and compatibility of packaging materials. The specification and requirement of quality testing depends on type of pharmaceutical materials used.Containers are tested by many methods of which commonly used test for glass are
- Crushed glass test
- Whole-Container test
- Chemical resistance of test
- Water Attack Test etc.
- Transparency test
- Penetrability
- Fragmentation test
- Self seal ability test,
- Extractive test etc.
Packaging is a process by which the pharmaceuticals are suitably packed so that they should retain their therapeutic effectiveness from the time of packaging till they are consumed. Packaging may be defined as the art and science which involves preparing the articles for transport, storage display and use. Pharmaceutical packaging is the means of providing protection, presentation, identification, information and convenience to encourage compliance with a course of therapy.
B. Closure
C. Carton or Outer
D. Box
1. Testing material
Tests applied to packaging materials may be:
2. Testing Packages
There are various tests to ensure that the resultant product will comply with its specification. Tests applied to the environment or to equipment, as well as to products in process, may also be regarded as a part of in-process control.
Containers must be chosen with care and after taking into consideration the nature of the articles and the likely effects of transportation and storage, even for short periods of time.
A container should be designed so that the contents may be removed in a manner suitable for the intended use of the article in it. It should also provide an adequate degree of protection, minimize the loss of constituents and should not interact physically or chemically with the contents in a way that will alter their quality to an extent beyond the limits given in the individual monograph, or present a risk of toxicity.
Components of Package:
A. ContainerB. Closure
C. Carton or Outer
D. Box
Procedure for Package Testing
It may be classified into two groups according to whether the test is applied to the packaging material in isolation or to the entire package.1. Testing material
Tests applied to packaging materials may be:
- Chemical - pH value of materials chloride and sulphate in paper or board, alkalinity of glass, compatibility test with chemicals or medicaments are typical of the chemical tests.
- Mechanical-Standard tests are available for the effect of creasing, folding and so on.
- Environmental-Materials may be tested by standard methods for absorption of water, permeability to water vapour, gases, oils, odours etc. and for characteristics such as light transmission.
2. Testing Packages
- Mechanical – Mechanical tests are applied mainly to outer packaging for protection from transportation hazards. They consist of the use of a standardized test procedure to compare the effect of different protective materials to prevent damage to the contents.
- Environmental- Packages are subjected to conditions that reproduce the environment and some evaluation is made at suitable intervals. Such procedures may be applied to testing closures for water vapour transmission.
Hazards Encountered by a Package
- Mechanical hazards – shock, compression, puncture, vibration etc.
- Environmental hazards-temperature, pressure, moisture, gases, light, contamination etc.
There are various tests to ensure that the resultant product will comply with its specification. Tests applied to the environment or to equipment, as well as to products in process, may also be regarded as a part of in-process control.
Principle instrumental techniques Employed For Packaging Controls
- Spectrophotometry
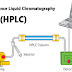
- Chromatographic Methods
- Thermal analysis techniques
- Gas transmission analysis
- Leak detection
- Physical test methods
- X-ray Fluorescence Analysis
Ideal Properties of Good Package
An ideal package should have following properties- Hold the product without loss on account of leakage, spoilage or permeation.
- Protect against environmental conditions like light, air and moisture during storage.
- Impermeable for gases.
- Sufficient strength to withstand shocks of handling, transportation etc.
- Facilitate efficient safe and convenient use of contents.
- Material must not interact with the contents.
- Containers should afford protection from moulds, bacteria etc.
- Cost of material should be as low as possible without compromising the quality.
- Allows easily identification of products.
- Afford protection from moulds, bacteria etc.
- Container should not absorb or adsorb any material containing.
- Closure should provide air tight closing to the container.
- Closure should be compatible with the product.
Quality Control of Containers
A container for a pharmacopoeial article is intended to contain a drug substance or drug product with which it is, or may be in direct contact. The closure is a part of the container.Containers must be chosen with care and after taking into consideration the nature of the articles and the likely effects of transportation and storage, even for short periods of time.
A container should be designed so that the contents may be removed in a manner suitable for the intended use of the article in it. It should also provide an adequate degree of protection, minimize the loss of constituents and should not interact physically or chemically with the contents in a way that will alter their quality to an extent beyond the limits given in the individual monograph, or present a risk of toxicity.